- Choosing a selection results in a full page refresh.
Need Help?
03444440083


Couldn't load pickup availability
standard shipping charges on order is Rs 200
You may return most new, unopened items within 3 days of delivery for a full refund.
You should expect to receive your refund within four weeks of giving your package to the return shipper, however, in many cases you will receive a refund more quickly. This time period includes the transit time for us to receive your return from the shipper (5 to 10 business days), the time it takes us to process your return once we receive it (3 to 5 business days), and the time it takes your bank to process our refund request (5 to 10 business days).
If you need to return an item, simply login to your account, view the order using the "Complete Orders" link under the My Account menu and click the Return Item(s) button. We'll notify you via e-mail of your refund once we've received and processed the returned item.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
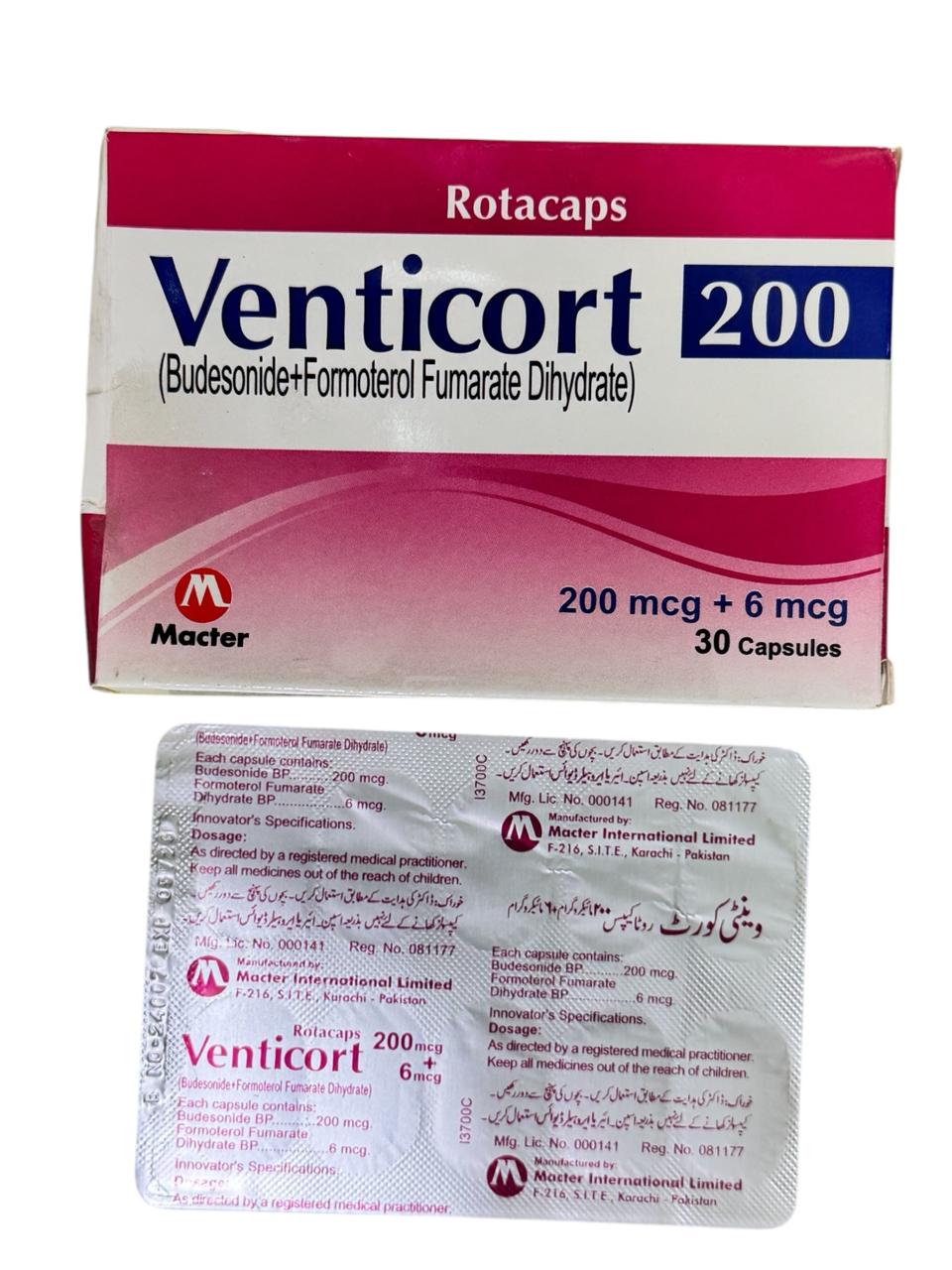
(1 box = 30 Capsules)
Specification
Requires Prescription (YES/NO)
Yes
Generics
Formoterol Fumarate, Budesonide
Used For
Asthma
How it works
Budesonide : Budesonide is a glucocorticosteroid which when inhaled has a dose-dependent antiinflammatory action in the airways, resulting in reduced symptoms and fewer exacerbations. Inhaled Budesonide has less severe adverse effects than systemic corticosteroids. The exact mechanism responsible for the anti-inflammatory effect of glucocorticosteroids is unknown.Formoterol : Formoterol is a selective ß2 adrenoceptor agonist which when inhaled results in rapid and long-acting relaxation of bronchial smooth muscle in patients with reversible airways obstruction. The bronchodilating effect is dose dependent, with an onset of effect within 1-3 minutes. The duration of effect is at least 12 hours after a single dose.
Usage And Safety
Dosage
Formoterol Fumarate, Budesonide
Side Effects
Common Candida infections in the oropharynx, headache, tremor, palpitations, mild irritation in the throat, coughing and hoarseness. Uncommon Aggression, psychomotor hyperactivity, anxiety, sleep disorders, dizziness, tachycardia, nausea, bruises and muscle cramps. Rare Immediate and delayed hypersensitivity reactions, e.g. exanthema, urticaria, pruritus, dermatitis, angioedema and anaphylactic reaction, hypokalemia, cardiac arrhythmias, e.g. atrial fibrillation, supraventricular tachycardia, extrasystoles and bronchospasm. Very Rare Cushing's syndrome, adrenal suppression, growth retardation, decrease in bone mineral density, hyperglycemia, depression, behavioural changes, taste disturbances, cataract, glaucoma, angina pectoris, prolongation of QTC-interval and variations in blood pressure
Drug Interactions
Potent inhibitors of CYP3A4 (e.g., ketoconazole, itraconazole, voriconazole, posaconazole, clarithromycin, telithromycin, nefazodone and HIV protease inhibitors) , ß blockers (including eye drops) , Concomitant treatment with quinidine, disopyramide, procainamide, phenothiazines and tricyclic antidepressants , L-Dopa, L-thyroxine, oxytocin and alcohol , monoamine oxidase inhibitors or tricyclic antidepressants,non-potassium-sparing diuretics (such as loop or thiazide diuretics).
Indication
It is indicated for the treatment of Asthma , Maintenance Treatment of Chronic Obstructive Pulmonary Disease (COPD).
When not to Use
Budesonide + Formoterol is contraindicated: - In patients with known hypersensitivity to Budesonide or Formoterol or to any excipient of the product. - In primary treatment of status asthmaticus or other acute episodes of asthma or COPD where intensive measures are required.
Precautions
Precaution
Glaucoma and cataracts have been reported in patients with asthma and COPD following the long-term administration of inhaled corticosteroids, including Budesonide. Therefore, close monitoring is warranted in patients with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts.
Warnings
Warning 1
Budesonide + Formoterol inhaler should not be initiated in patients during rapidly deteriorating or potentially life-threatening episodes of asthma or COPD. It should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm.
Warning 2
Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs. Patients using Budesonide + Formoterol inhaler should not use additional LABA (e.g., salmeterol, formoterol fumarate, arformoterol tartrate) for any reason, including prevention of exercise-induced bronchospasm (EIB) or the treatment of asthma or COPD.
Warning 3
It is possible that systemic corticosteroid effects such as hypercorticism and adrenal suppression (including adrenal crisis) may appear with very high dosages or at the regular dosage in susceptible individuals. If such changes occur, discontinue Budesonide + Formoterol inhaler slowly.
Additional Information
Pregnancy category
Always consult your physician before using any medicine.
Storage (YES/NO)
Store this medicine at room temperature, away from direct light and heat.
Get the latest updates on new products and upcoming sales
Thanks for subscribing!
This email has been registered!

| Product | SKU | Rating | Description | Collection | Availability | Product Type | Other Details |
|---|