- Choosing a selection results in a full page refresh.
Need Help?
03444440083


Couldn't load pickup availability
standard shipping charges on order is Rs 200
You may return most new, unopened items within 3 days of delivery for a full refund.
You should expect to receive your refund within four weeks of giving your package to the return shipper, however, in many cases you will receive a refund more quickly. This time period includes the transit time for us to receive your return from the shipper (5 to 10 business days), the time it takes us to process your return once we receive it (3 to 5 business days), and the time it takes your bank to process our refund request (5 to 10 business days).
If you need to return an item, simply login to your account, view the order using the "Complete Orders" link under the My Account menu and click the Return Item(s) button. We'll notify you via e-mail of your refund once we've received and processed the returned item.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
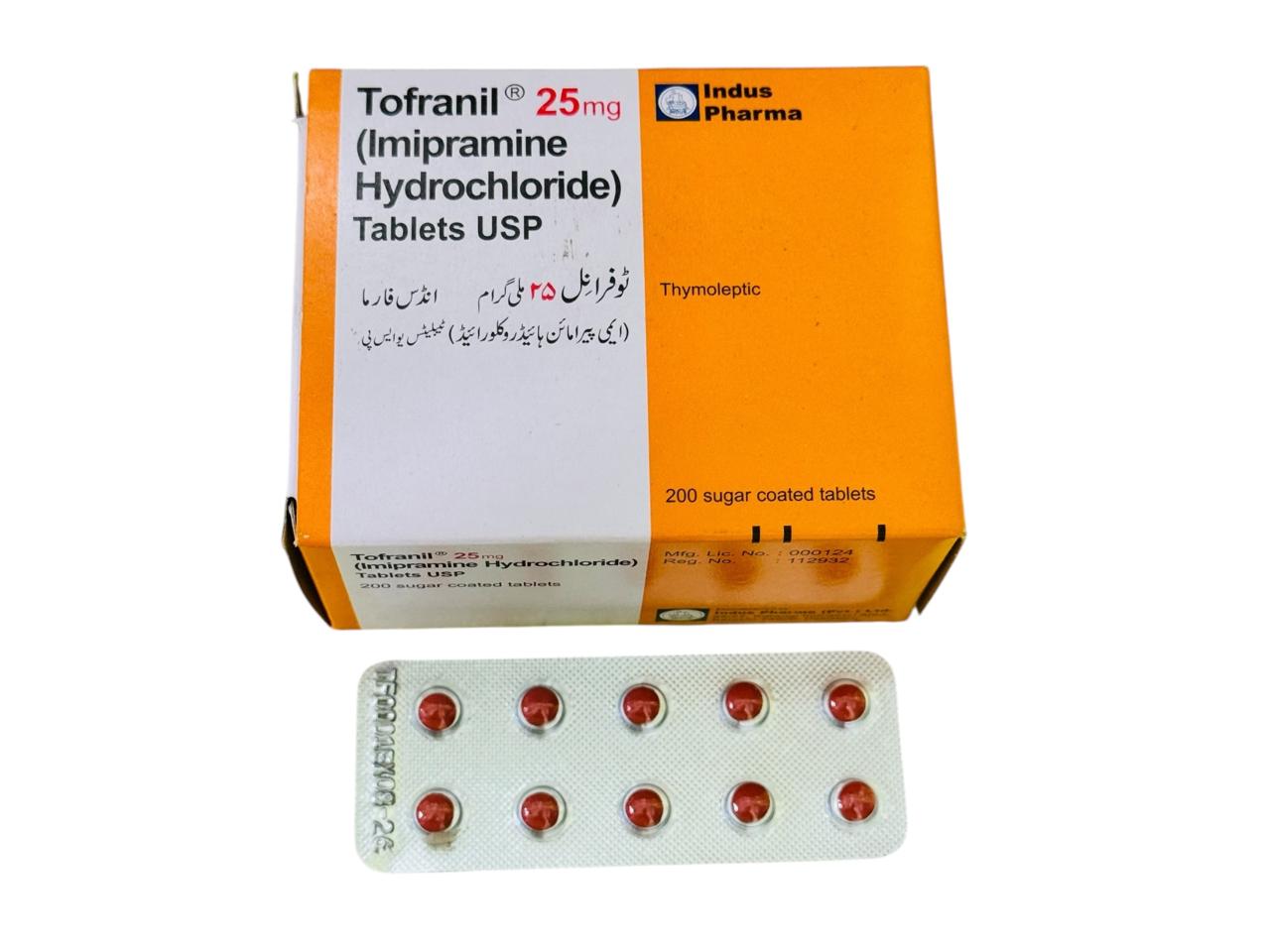
(1 Box = 200 Tablets)
Yes
Imipramine Hydrochloride
Depression
The pharmacological properties of imipramine are similar to those of other tricyclic antidepressants. The pharmacological profile of imipramine includes reversal of reserpine and tetrabenazine effects, slight depressant effects on the central nervous system as manifested by behavioral, motor and electrocortical visceral activity, anticholinergic and antihistaminic effects, and potentiation of adrenergic and serotonergic functions.
Imipramine Hydrochloride
Behavioral Common: Somnolence, fatigue, insomnia, confusional states with hallucinations (particularly in geriatric patients and patients suffering from Parkinson’s disease), anxiety, agitation, restlessness, nightmares, hypomania, mania, decrease in memory, feeling of unreality, disorientation. Rare: psychotic disorders. Very rare: Aggression. Anticholinergic Very common: Dry mouth and rarely associated sublingual adenitis, blurred vision, disturbances of visual accommodation, lacrimation decreased, constipation, hyperhidrosis, hot flushes. Common: micturition disorders, dilation of the urinary tract. Very rare: mydriasis, glaucoma, paralytic ileus, urinary retention. Cardiovascular Very common: hypotension, particularly orthostatic hypotension with associated vertigo, sinus tachycardia, electrocardiogram abnormalities (including flattening or inversion of T wave, depressed S-T segments). Common: Arrhythmia, disturbances in cardiac conduction (e.g., widening of QRS complex, PQ changes, bundle-branch block), palpitation, syncope. Very rare: hypertension, congestive heart failure, myocardial infarction, heart block, asystole, stroke, vasospams, QT interval prolongation, ventricular arrhythmia, ventricular tachycardia, ventricular fibrillation, torsades de pointes. Hematologic Very rare: agranulocytosis, eosinophilia, leukopenia, purpura and thrombocytopenia may occur as an idiosyncratic response. Gastrointestinal Common: nausea, vomiting, anorexia, abdominal cramps, liver function test abnormal. Rare: diarrhea .
CNS depressants (e.g., barbiturates, benzodiazepines or general anesthetics) or anticholinergic agents (e.g., atropine, antihistamines, biperiden, levodopa) , anti-arrhythmic agents , antihypertensives ( e.g. guanethidine, bethanidine, clonidine, reserpine or alpha-methyldopa, diuretics, vasodilators, beta-blockers) , noradrenaline or adrenaline, amphetamine ,cardiovascular drugs like noradrenaline or adrenaline,amphetamine , as well as nasal drops and local anesthetics containing sympathomimetics (e.g., isoprenaline, ephedrine, phenylephrine) , SSRIs such as fluoxetine, paroxetine, sertraline or citalopram are inhibitors of CYP2D6. Fluvoxamine is a potent inhibitor of CYP1A2 and a moderate inhibitor of CYP2D6 , serotonergic co-medications such as SSRIs, SNRIs, tricyclic antidepressants or lithium ; cimetidine or methylphenidate , Substances which activate the hepatic mono-oxygenase enzyme system (e.g., barbiturates, carbamazepine, phenytoin, nicotine and oral contraceptives , MAO-inhibitors , neuroleptic agents (e.g., phenothiazines and butyrophenones) , Oral antifungal, terbinafine.
It is indicated for the relief of symptoms of depression.
It is contraindicated in patients who have known or suspected hypersensitivity to the drug or its excipients, or have known or suspected hypersensitivity to tricyclic antidepressants belonging to the dibenzazepine group ; It should not be given in conjunction with, or within fourteen days before or after treatment with a monoamine oxidase (MAO) inhibitor ; The concomitant treatment with selective, reversible MAO-A inhibitors, such as moclobemide, is also contraindicated ; Symptoms such as hypertensive crises, hyperactivity, hyperpyrexia, spasticity, severe convulsions, and those consistent with serotonin syndrome, e.g. myoclonus, agitation, seizures, delirium or coma and death have been reported in patients receiving such combinations ; It is contraindicated for use during the acute recovery phase following a myocardial infarction and in the presence of acute congestive heart failure ; It is contraindicated in patients with existing liver or kidney damage and should not be administered to patients with a history of blood dyscrasias ; It is contraindicated in patients with glaucoma, as the condition may be aggravated due to the atropine-like effects of the drug .
The possibility of a suicide attempt is inherent in depression. These patients should be carefully supervised during treatment with imipramine hydrochloride and hospitalization or concomitant electroconvulsive therapy may be required. Prescriptions for Imipramine Hydrochloride should be written for the smallest possible quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Tricyclic agents are known to lower the convulsive threshold and imipramine hydrochloride should, therefore, be used with extreme caution in patients with a history of convulsive disorders and other predisposing factors, e.g., brain damage of varying etiology, concomitant use of neuroleptics, alcoholism and withdrawal from alcohol, and concomitant use with other drugs that lower the seizure threshold. It appears that the occurrence of seizures is dose dependent. Therefore, the recommended total daily doses should not be exceeded .
Due to the risk of serotonergic toxicity, it is advisable to adhere to recommended doses and any increase in dose should be made with caution if other serotonergic agents are co-administered. Serotonin syndrome, with symptoms such as hyperpyrexia, myoclonus, agitation, seizures, delirium and coma, can possibly occur when imipramine is administered with serotonergic co- medications such as selective serotonin reuptake inhibitors (SSRIs), serotonin and noradrenaline reuptake inhibitors (SNRIs), tricyclic antidepressants or lithium .
Caution should be observed when prescribing Imipramine Hydrochloride for hyperthyroid patients or for patients receiving thyroid medication. Transient cardiac arrhythmias have occurred in rare instances in patients who have been receiving other tricyclic compounds concomitantly with thyroid medication.
Always consult your physician before using any medicine.
Store this medicine at room temperature, away from direct light and heat.
Get the latest updates on new products and upcoming sales
Thanks for subscribing!
This email has been registered!

| Product | SKU | Rating | Description | Collection | Availability | Product Type | Other Details |
|---|