- Choosing a selection results in a full page refresh.
Need Help?
03444440083


Couldn't load pickup availability
standard shipping charges on order is Rs 200
You may return most new, unopened items within 3 days of delivery for a full refund.
You should expect to receive your refund within four weeks of giving your package to the return shipper, however, in many cases you will receive a refund more quickly. This time period includes the transit time for us to receive your return from the shipper (5 to 10 business days), the time it takes us to process your return once we receive it (3 to 5 business days), and the time it takes your bank to process our refund request (5 to 10 business days).
If you need to return an item, simply login to your account, view the order using the "Complete Orders" link under the My Account menu and click the Return Item(s) button. We'll notify you via e-mail of your refund once we've received and processed the returned item.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
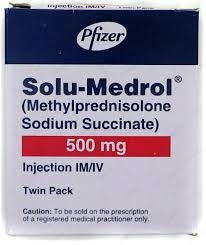
Specification
Requires Prescription (YES/NO)
Yes
Generics
Methyl Prednisolone Sodium Succinate
Used For
Allergy
How it works
Methylprednisolone is a potent anti-inflammatory steroid with greater anti-inflammatory potency than prednisolone and even less tendency than prednisolone to induce sodium and water retention.
Usage And Safety
Dosage
Methyl Prednisolone Sodium Succinate
Side Effects
Gastrointestinal: Abdominal distention, bowel/bladder dysfunction (after intrathecal administration), elevation in serum liver enzyme levels (usually reversible upon discontinuation), hepatomegaly, increased appetite, nausea, pancreatitis, peptic ulcer with possible perforation and hemorrhage, perforation of the small and large intestine (particularly in patients with inflammatory bowel disease), ulcerative esophagitis. Metabolic: Negative nitrogen balance due to protein catabolism. Musculoskeletal: Aseptic necrosis of femoral and humeral heads, Charcot-like arthropathy, loss of muscle mass, muscle weakness, osteoporosis, pathologic fracture of long bones, postinjection flare (following intra-articular use), steroid myopathy, tendon rupture, vertebral compression fractures. Neurologic/Psychiatric: Convulsions, depression, emotional instability, euphoria, headache, increased intracranial pressure with papilledema (pseudotumor cerebri) usually following discontinuation of treatment, insomnia, mood swings, neuritis, neuropathy, paresthesia, personality changes, psychic disorders, vertigo. Arachnoiditis, meningitis, paraparesis/paraplegia, and sensory disturbances have occurred after intrathecal administration . Ophthalmic: Exophthalmos, glaucoma, increased intraocular pressure, posterior subcapsular cataracts, rare instances of blindness associated with periocular injections. Other: Abnormal fat deposits, decreased resistance to infection, hiccups, increased or decreased motility and number of spermatozoa, injection site infections following nonsterile administration , malaise, moon face, weight gain.
Drug Interactions
Live vaccines or other vaccines , anticoagulants , hypoglycaemia agents , antihypertensives , diuretics , erythromycin , rifamycin , amphotericin , azole antifungals , ritonavir , cyclosporin , cardiac glycosides . Oestrogens including oral contraceptives , somatropin . Carbamazepine , phenytoin , primidone , barbiturates . Muscle relaxants , NSAIDs , salicylates , sympathomimetics , mifepristone , methotrexate , theophylline , aprepitant .
Indication
It is used for sensitivity reactions , transplantation , cerebral oedema , secondary to tumour , ulcerative colitis , crohn's disease , aspiration of gastric contents , stevens-johnson syndrome .
When not to Use
It is contraindicated: • In systemic fungal infections and patients with known hypersensitivity to the product and its constituents. • For intrathecal administration. Reports of severe medical events have been associated with this route of administration.
Precautions
Precaution
Thrombosis including venous thromboembolism has been reported to occur with corticosteroids. As a result corticosteroids should be used with caution in patients who have or may be predisposed to thromboembolic disorders.
Warnings
Warning 1
Dosage must be decreased or discontinued gradually when the drug has been administered for more than a few days.
Warning 2
Adverse effects of glucocorticoids on the cardiovascular system, such as dyslipidemia and hypertension may predispose treated patients with existing cardiovascular risk factors to additional cardiovascular effects, if high doses and prolonged courses are used. Accordingly, corticosteroids should be employed judiciously in such patients and attention should be paid to risk modification and additional cardiac monitoring if needed.
Warning 3
Aspirin (acetylsalicylic acid) should be used cautiously in conjunction with corticosteroids in hypoprothrombinemia.
Additional Information
Pregnancy category
Always consult your physician before using any medicine.
Storage (YES/NO)
Store this medicine at room temperature, away from direct light and heat.
Get the latest updates on new products and upcoming sales
Thanks for subscribing!
This email has been registered!

| Product | SKU | Rating | Description | Collection | Availability | Product Type | Other Details |
|---|