- Choosing a selection results in a full page refresh.
Need Help?
03444440083


Couldn't load pickup availability
standard shipping charges on order is Rs 200
You may return most new, unopened items within 3 days of delivery for a full refund.
You should expect to receive your refund within four weeks of giving your package to the return shipper, however, in many cases you will receive a refund more quickly. This time period includes the transit time for us to receive your return from the shipper (5 to 10 business days), the time it takes us to process your return once we receive it (3 to 5 business days), and the time it takes your bank to process our refund request (5 to 10 business days).
If you need to return an item, simply login to your account, view the order using the "Complete Orders" link under the My Account menu and click the Return Item(s) button. We'll notify you via e-mail of your refund once we've received and processed the returned item.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
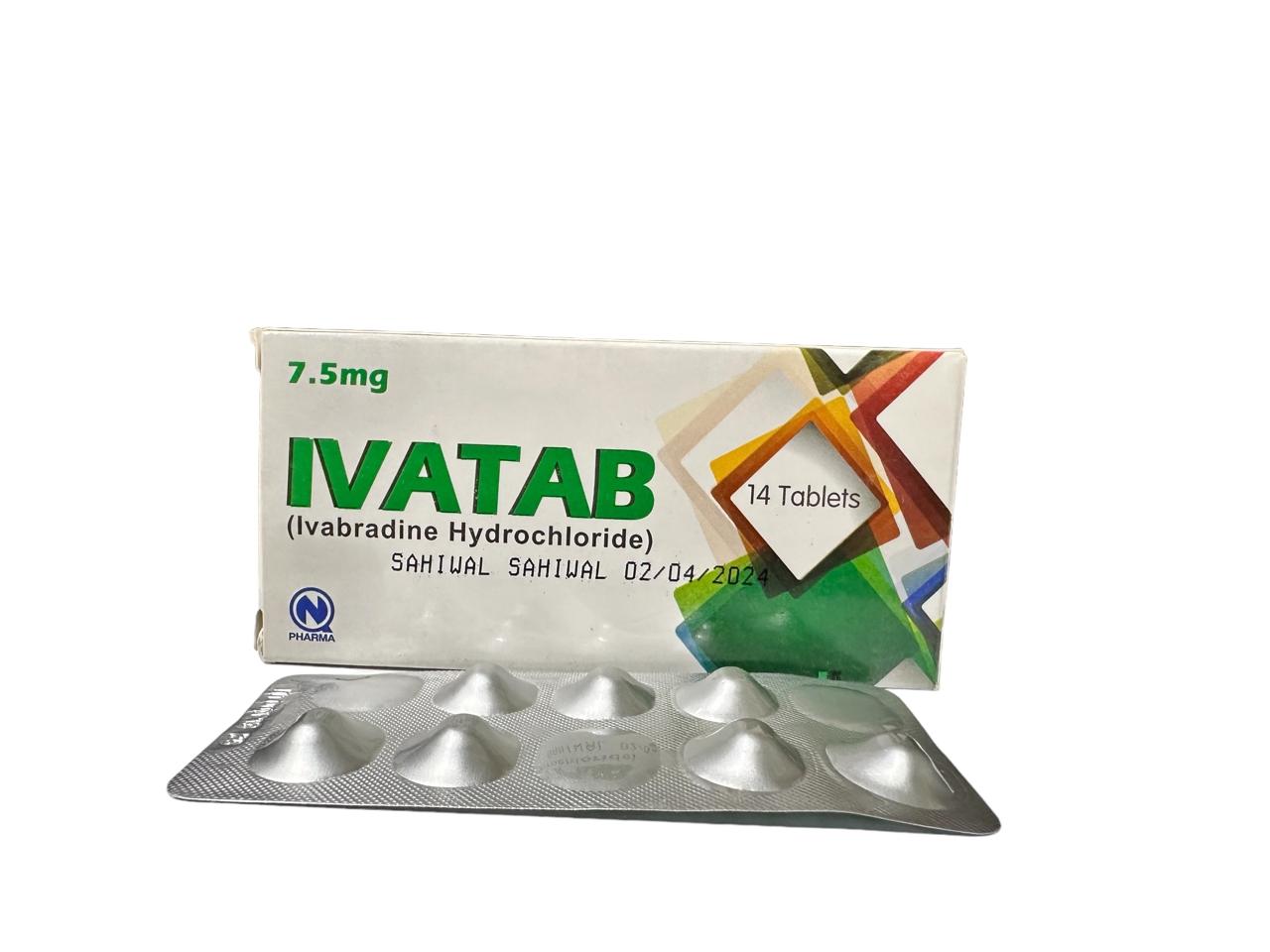
Ivatab 7.5mg Tablet 1 Box= 2 Strips.
Specification
Requires Prescription (YES/NO)
Yes
Generics
Ivabradine HCl
Used For
Angina
How it works
Ivabradine is a pure heart rate lowering agent, acting by selective and specific inhibition of the cardiac pacemaker If current that controls the spontaneous diastolic depolarization in the sinus node and regulates heart rate. The cardiac effects are specific to the sinus node with no effect on intra-atrial, atrioventricular or intraventricular conduction times, nor on myocardial contractility or ventricular repolarization.
Usage And Safety
Dosage
Ivabradine HCl
Side Effects
The most common adverse effects seen with ivabradine are luminous phenomena in the visual field (Phosphenes). Other adverse reactions include blurred vision, bradycardia, which may be severe and other cardiac arrhythmias, nausea, constipation, diarrhea, headache, dizziness, dyspnea and muscle cramps. Hyperuricemia, eosinophilia and elevated blood creatinine concentrations have been reported.
Drug Interactions
Concomitant use with Cytochrome P450 3A4 (CYP3A4) inhibitor or inducers: CYP3A4 inhibitors increase ivabradine plasma concentrations, while inducers decrease them. Increased plasma concentrations of ivabradine may be associated with a risk of excessive bradycardia.- Moderate CYP3A4 inhibitors (e.g. diltiazem, verapamil) with heart rate reducing properties: Concomitant use of ivabradine with diltiazem and verapamil is not recommended due to the potential for additive heart rate lowering effects.- Other moderate CYP3A4 inhibitors: Ivabradine can be used with caution if resting heart rate is at or above 60 bpm and heart rate is carefully monitored.- Grapefruit Juice: Grapefruit juice increase ivabradine exposure. Therefore, the intake of grapefruit juice should be restricted during the treatment with ivabradine.- CYP3A4 inducers (e.g. rifampicin, barbiturates, phenytoin, St John's Wort): Prolonged concomitant administration of these agents with ivabradine may decrease ivabradine exposure and therefore require an adjustment of dose depending upon the therapeutic response.Concomitant use with QT-prolonging medicines: The concomitant use of cardiovascular (e.g. quinidine, disopyramide, sotalol, amiodarone) or non-cardiovascular (e.g. tricyclic antidepressants, antipsychotics, erythromycin IV, pentamidine, pimozide, mefloquine) QT prolonging medicines with ivabradine should be avoided since QT prolongation may be exacerbated by heart rate reduction. If combination appears necessary, close cardiac monitoring is needed.Concomitant use with Potassium-depleting diuretics: Hypokalemia can increase the risk of arrhythmia. As ivabradine may cause bradycardia, the resulting combination of hypokalemia and bradycardia is a predisposing factor to the onset of severe arrhythmias, especially in patients with long QT syndrome, whether congenital or substance-induced.
Indication
Coronory Artery Disease (CAD) : Symptomatic treatment of chronic stable angina pectoris in coronary artery disease adults with normal sinus rhythm:- Who are unable to tolerate or have a contraindication to beta blockers, or- In combination with beta-blockers in patients inadequately controlled with an optimal beta-blocker dose and whose heart rate is > 60 bpm . Chronic Heart Failure (CHF) : Symptomatic treatment of chronic heart failure of NYHA Classes II or III and with documented left ventricular ejection fraction (LVEF) = 35% in adult patients in sinus rhythm and with heart rate at or above 77 bpm, in combination with optimal standard chronic heart failure treatment.
When not to Use
Ivabradine is contraindicated in patients with: - Hypersensitivity to ivabradine or to any of the excipient of the product - Resting heart rate below 60 bpm prior to treatment- Cardiogenic shock - Acute myocardial infarction - Severe hypotension (< 90/50 mm/Hg) - Severe hepatic impairment- Sick sinus syndrome - Sino-atrial block - Unstable or acute heart failure - Pacemaker dependent - Unstable angina- AV-block 3rd degree- Combination with strong cytochrome P450 3A4 inhibitors such as azole antifungals (ketoconozole, itraconozole), macrolide antibiotics (clarithromycin, erythromycin, josamycin, telithomycin), HIV protease inhibitors (nelfinavir, ritonavir) and nefazodone. - Pregnancy, nursing mothers and women of child-bearing potential not using appropriate contraceptive measure.
Precautions
Precaution
Cardiac arrhythmias: Ivabradine is not recommended in patients with atrial fibrillation or other cardiac arrhythmias that interfere with sinus node function. It is recommended to regularly clinically monitor ivabradine treated patients for the occurrence of atrial fibrillation which should also include ECG monitoring if clinically indicated. The risk of developing atrial fibrillation may be higher in chronic heart failure patients treated with ivabradine. Chronic heart failure patients with intraventricular conduction defects and ventricular dyssynchrony should be monitored closely .
Warnings
Warning 1
Low heart rate: If during treatment, resting heart rate decreases persistently below 50 bpm or the patient experiences symptoms related to bradycardia such as dizziness, fatigue or hypotension, the dose must be titrated downward or treatment discontinued if heart rate below 50 bpm or symptoms of bradycardia persist.
Warning 2
Chronic heart failure: Heart failure must be stable before considering ivabradine treatment. Ivabradine should be used with caution in heart failure patients with NYHA functional classification IV .
Warning 3
Hypotension: : Ivabradine should be used with caution in patients with mild to moderate hypotension .
Additional Information
Pregnancy category
Always consult your physician before using any medicine.
Storage (YES/NO)
Store this medicine at room temperature, away from direct light and heat.
Get the latest updates on new products and upcoming sales
Thanks for subscribing!
This email has been registered!

| Product | SKU | Rating | Description | Collection | Availability | Product Type | Other Details |
|---|