- Choosing a selection results in a full page refresh.
Need Help?
03444440083


Couldn't load pickup availability
standard shipping charges on order is Rs 200
You may return most new, unopened items within 3 days of delivery for a full refund.
You should expect to receive your refund within four weeks of giving your package to the return shipper, however, in many cases you will receive a refund more quickly. This time period includes the transit time for us to receive your return from the shipper (5 to 10 business days), the time it takes us to process your return once we receive it (3 to 5 business days), and the time it takes your bank to process our refund request (5 to 10 business days).
If you need to return an item, simply login to your account, view the order using the "Complete Orders" link under the My Account menu and click the Return Item(s) button. We'll notify you via e-mail of your refund once we've received and processed the returned item.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
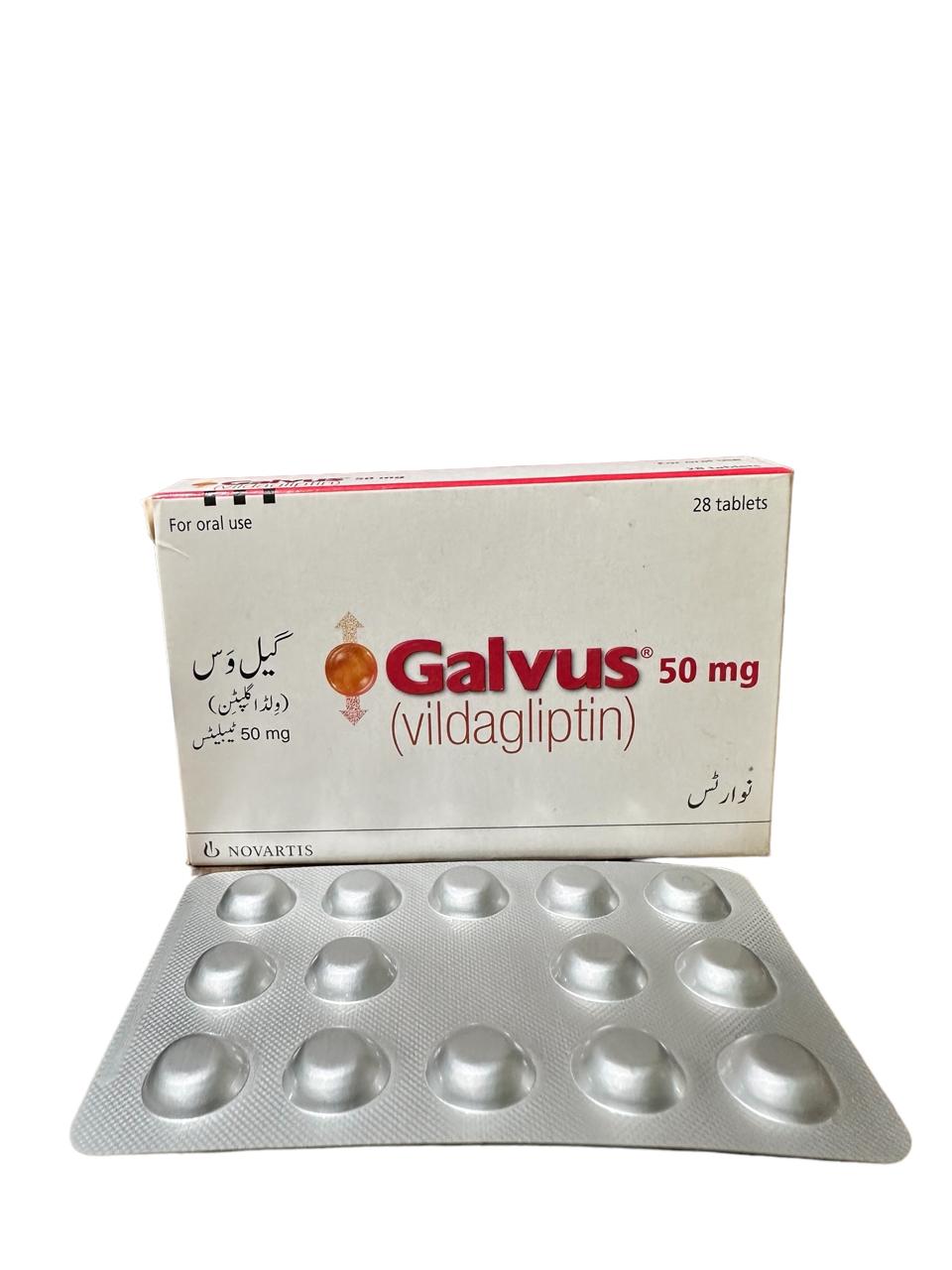
Galvus Tablets 50Mg (1 Box = 2 Strips)
Specification
Requires Prescription (YES/NO)
Yes
Generics
Vildagliptin
Used For
Diabetes
How it works
The administration of vildagliptin results in a rapid and complete inhibition of DPP-4 activity, resulting in increased fasting and postprandial endogenous levels of the incretin hormones GLP-1 (glucagon-like peptide 1) and GIP (glucose-dependent insulinotropic polypeptide).
Usage And Safety
Dosage
Vildagliptin
Side Effects
Monotherapy Common: Dizziness. Uncommon: Hypoglycemia, headache, edema peripheral, constipation & arthralgia. Very Rare: Upper respiratory tract infection & nasopharyngitis. Combination with Metformin Common: Hypoglycemia, tremor, headache, dizziness & nausea. Uncommon: Fatigue. Combination with Sulfonylurea Common: Hypoglycemia, tremor, headache, dizziness & asthenia. Uncommon: Constipation. Very Rare: Nasopharyngitis. Combination with Thiazolidinedione Common: Weight increase & edema peripheral. Uncommon: Hypoglycemia, headache & asthenia. Combination with Metformin and Sulfonylurea Common: Hypoglycemia, dizziness, tremor, hyperhidrosis & asthenia. Combination with Insulin Common: Decreased blood glucose, headache, chills, nausea, gastroesophageal reflux disease. Uncommon: Diarrhea & flatulence.
Drug Interactions
Vildagliptin has a low potential for interactions with co-administered medicinal products. Since vildagliptin is not a cytochrome P (CYP) 450 enzyme substrate and does not inhibit or induce CYP450 enzymes, it isnot likely to interact with active substances that are substrates, inhibitors or inducers of these enzymes. As with other oral antidiabetic medicinal products the hypoglycemic effect of vildagliptin may be reduced by certain active substances, including thiazides, corticosteroids, thyroid products and sympathomimetics.
Indication
Vildagliptin is indicated for the treatment of type 2 diabetes mellitus in adults:As monotherapy - In patients inadequately controlled by diet and exercise alone and for whom metformin is inappropriate due to contraindications or intolerance.As dual oral therapy in combination with - Metformin, in patients with insufficient glycemic control despite maximal tolerated dose of monotherapy with metformin.- Sulfonylurea, in patients with insufficient glycemic control despite maximal tolerated dose of a sulfonylurea and for whom metformin is inappropriate due to contraindications or intolerance.- Thiazolidinedione, in patients with insufficient glycemic control and for whom the use of a thiazolidinedione is appropriate. As triple oral therapy in combination with- Sulfonylurea and metformin when diet and exercise plus dual therapy with these medicinal products do not provide adequate glycemic control.Vildagliptin is also indicated for use in combination with insulin (with or without metformin) when diet and exercise plus a stable dose of insulin do not provide adequate glycemic control.
When not to Use
Vildagliptin is contraindicated in patients with known hypersensitivity to vildagliptin or to any excipient of the product.
Precautions
Precaution
Rare cases of hepatic dysfunction (including hepatitis) have been reported. Liver function tests should be performed prior to the initiation of treatment. Liver function should be monitored during treatment with vildagliptin at three-month intervals during the first year and periodically thereafter. Patients who develop jaundice or other signs suggestive of liver dysfunction should discontinue vildagliptin.
Warnings
Warning 1
Vildagliptin should not be used in patients with type 1 diabetes or for the treatment of diabetic ketoacidosis.
Warning 2
In diabetic patient, monitoring for skin disorders, such as blistering or ulceration, is recommended.
Warning 3
Use of vildagliptin has been associated with a risk of developing acute pancreatitis. If pancreatitis is suspected, vildagliptin should be discontinued and if acute pancreatitis is confirmed, vildagliptin should not be restarted. Caution should be exercised in patients with a history of acute pancreatitis.
Additional Information
Pregnancy category
Always consult your physician before using any medicine.
Storage (YES/NO)
Store this medicine at room temperature, away from direct light and heat.
Get the latest updates on new products and upcoming sales
Thanks for subscribing!
This email has been registered!

| Product | SKU | Rating | Description | Collection | Availability | Product Type | Other Details |
|---|