
- Choosing a selection results in a full page refresh.
Need Help?
03444440083


Couldn't load pickup availability
standard shipping charges on order is Rs 200
You may return most new, unopened items within 3 days of delivery for a full refund.
You should expect to receive your refund within four weeks of giving your package to the return shipper, however, in many cases you will receive a refund more quickly. This time period includes the transit time for us to receive your return from the shipper (5 to 10 business days), the time it takes us to process your return once we receive it (3 to 5 business days), and the time it takes your bank to process our refund request (5 to 10 business days).
If you need to return an item, simply login to your account, view the order using the "Complete Orders" link under the My Account menu and click the Return Item(s) button. We'll notify you via e-mail of your refund once we've received and processed the returned item.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
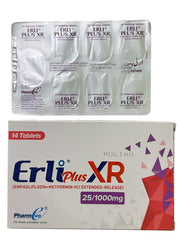
(1 Box = 2 Strips )
Specification
Requires Prescription (YES/NO)
Yes
Generics
Empagliflozin , Metformin HCl
Used For
Diabetes
How it works
Metformin hydrochloride is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes mellitus, lowering both basal and postprandial plasma glucose. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. Empagliflozin Sodium-glucose co-transporter 2 (SGLT2) is the predominant transporter responsible for reabsorption of glucose from the glomerular filtrate back into the circulation. Empagliflozin is an inhibitor of SGLT2. By inhibiting SGLT2, Empagliflozin reduces renal reabsorption of filtered glucose and lowers the renal threshold for glucose, and thereby increases urinary glucose excretion.
Usage And Safety
Dosage
Empagliflozin , Metformin HCl
Side Effects
Most common adverse reactions associated with empagliflozin (5% or greater incidence) were urinary tract infection and female genital mycotic infections. Most common adverse reactions associated with metformin (>5%) are diarrhea, nausea/vomiting, flatulence, abdominal discomfort, indigestion, asthenia, and headache.
Drug Interactions
Diuretics , Positive Urine Glucose Test , Interference with 1,5-anhydroglucitol (1,5-AG) Assay , ranolazine, vandetanib, dolutegravir, and cimetidine , zonisamide, acetazolamide or dichlorphenamide , corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blocking drugs, and isoniazid , Nifedipine
Indication
It is a combination of empagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor and metformin, a biguanide, indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus who are not adequately controlled on a regimen containing empagliflozin or metformin, or in patients already being treated with both empagliflozin and metformin.
When not to Use
It should not be used when Renal Impairment, ESRD, or on dialysis, Metabolic acidosis, including diabetic ketoacidosis, History of serious hypersensitivity reaction to empagliflozin or metformin.
Precautions
Precaution
Keep all medicines out of the reach and sight of children. Store in a cool, dry place, away from direct heat and light.
Warnings
Warning 1
Lactic Acidosis: There have been postmarketing cases of Metformin-associated lactic acidosis, including fatal cases. These cases had a subtle onset and were accompanied by nonspecific symptoms such as malaise, myalgias, abdominal pain, respiratory distress, or increased somnolence; however, hypothermia, hypotension, and resistant bradyarrhythmias have occurred with severe acidosis.
Warning 2
Hypotension: Empagliflozin causes intravascular volume contraction. Symptomatic hypotension may occur after initiating Empagliflozin particularly in patients with renal impairment, the elderly, in patients with low systolic blood pressure, and in patients on diuretics. Before initiating this medicine , assess for volume contraction and correct volume status if indicated. Monitor for signs and symptoms of hypotension after initiating therapy and increase monitoring in clinical situations where volume contraction is expected.
Warning 3
Ketoacidosis: Ketoacidosis, a serious life-threatening condition require urgent hospitalization in patients with type 1 and type 2 diabetes mellitus receiving sodium glucose co-transporter-2 (SGLT2) inhibitors, including Empagliflozin. Fatal cases of ketoacidosis have been reported in patients taking Empagliflozin.These medicines are not indicated for the treatment of patients with type 1 diabetes mellitus.
Additional Information
Pregnancy category
Always consult your physician before using any medicine.
Storage (YES/NO)
Store this medicine at room temperature, away from direct light and heat.
Get the latest updates on new products and upcoming sales
Thanks for subscribing!
This email has been registered!

| Product | SKU | Rating | Description | Collection | Availability | Product Type | Other Details |
|---|