- Choosing a selection results in a full page refresh.
Need Help?
03444440083


Couldn't load pickup availability
standard shipping charges on order is Rs 200
You may return most new, unopened items within 3 days of delivery for a full refund.
You should expect to receive your refund within four weeks of giving your package to the return shipper, however, in many cases you will receive a refund more quickly. This time period includes the transit time for us to receive your return from the shipper (5 to 10 business days), the time it takes us to process your return once we receive it (3 to 5 business days), and the time it takes your bank to process our refund request (5 to 10 business days).
If you need to return an item, simply login to your account, view the order using the "Complete Orders" link under the My Account menu and click the Return Item(s) button. We'll notify you via e-mail of your refund once we've received and processed the returned item.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
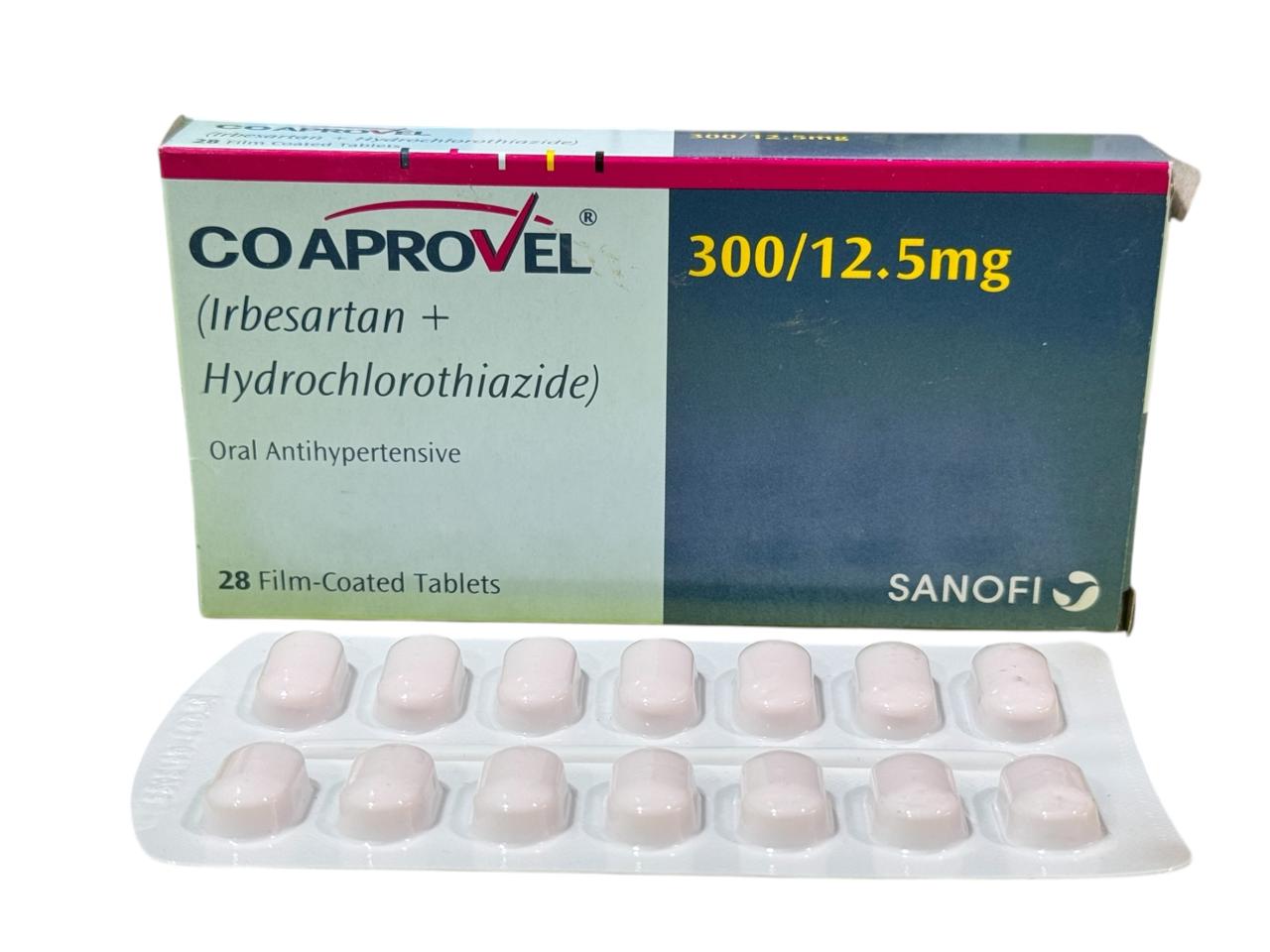
1 Box = 28 Tablets
Specification
Requires Prescription (YES/NO)
Yes
Generics
Irbesartan , Hydrochlorothiazide
Used For
Hypertension
How it works
Irbesartan : Irbesartan is a potent, orally active, selective angiotensin-II receptor (type AT1) antagonist. It is expected to block all actions of angiotensin-II mediated by the AT1 receptor, regardless of the source or route of synthesis of angiotensin-II. The selective antagonism of the angiotensin-II (AT1) receptors results in increases in plasma renin levels and angiotensin-II levels, and a decrease in plasma aldosterone concentration. Serum potassium levels are not significantly affected by irbesartan alone at the recommended doses. Irbesartan does not inhibit ACE (kininase-II), an enzyme which generates angiotensin-II and also degrades bradykinin into inactive metabolites. Irbesartan does not require metabolic activation for its activity. Hydrochlorothiazide : Hydrochlorothiazide is a thiazide diuretic. The mechanism of antihypertensive effect of thiazide diuretic is not fully known. Thiazides affect the renal tubular mechanisms of electrolyte reabsorption, directly increasing excretion of sodium and chloride in approximately equivalent amounts. The diuretic action of hydrochlorothiazide reduces plasma volume, increases plasma renin activity, increases aldosterone secretion, with consequent increases in urinary potassium and bicarbonate loss, and decreases in serum potassium. Presumably through blockade of the renin-angiotensin-aldosterone system, co-administration of irbesartan tends to reverse the potassium loss associated with these diuretics. With hydrochlorothiazide, onset of diuresis occurs in 2 hours, and peak effect occurs at about 4 hours, while the action persists for approximately 6-12 hours.
Usage And Safety
Dosage
Irbesartan , Hydrochlorothiazide
Side Effects
Cardiac disorders: Common: increases in blood urea nitrogen (BUN), creatinine and creatine kinase Uncommon: decreases in serum potassium and sodium .Uncommon: syncope, hypotension, tachycardia, oedema.Nervous system disorders: Common: dizziness Uncommon: orthostatic dizziness Not known: headache . Ear and labyrinth disorders: Not known: tinnitus. Respiratory, thoracic and mediastinal disorders: Not known: cough. Gastrointestinal disorders: Common: nausea/vomiting , Uncommon: diarrhoea , Not known: dyspepsia, dysgeusia .
Drug Interactions
Irbesartan : Lithium , kaliuretic diuretics, laxatives, amphotericin, carbenoxolone, penicillin G sodium , heparin sodium , digitalis glycosides, antiarrhythmics , selective COX-2 inhibitors, acetylsalicylic acid , Hydrochlorothiazide: Thiazide diuretics , Alcohol , Antidiabetic medicinal products (oral agents and insulins) , Colestyramine and Colestipol resins , Corticosteroids, ACTH , Digitalis glycoside , Pressor amines (e.g. noradrenaline) , tubocurarine , probenecid or sulfinpyrazone , calcium supplements or calcium sparing medicinal products (e.g. vitamin D therapy) , Carbamazepine , atropine, beperiden , cyclophosphamide, methotrexate.
Indication
• Treatment of essential hypertension. • This fixed dose combination is indicated in adult patients whose blood pressure is not adequately controlled on irbesartan or hydrochlorothiazide alone. • It may also be used as initial therapy in patients who are likely to need multiple drugs to achieve their blood pressure goals .
When not to Use
• Hypersensitivity to the active substances or to any of the excipients or to other sulfonamide-derived substances (hydrochlorothiazide is a sulfonamide-derived substance)• Second and third trimesters of pregnancy.• Severe renal impairment (creatinine clearance <30 ml/min).• Refractory hypokalaemia, hypercalcaemia.• Severe hepatic impairment, biliary cirrhosis and cholestasis.• The concomitant use of Irbesartan/Hydrochlorothiazide with aliskiren-containing products is contraindicated in patients with diabetes mellitus or renal impairment (creatinine clearance <60ml/min/1.73 m²).• Due to its HCT component Irbesartan/HCT tablets are contraindicated in anuric patients
Precautions
Precaution
It has been rarely associated with symptomatic hypotension in hypertensive patients without other risk factors for hypotension. Symptomatic hypotension may be expected to occur in patients who are volume and/or sodium depleted by vigorous diuretic therapy, dietary salt restriction, diarrhea or vomiting. Such conditions should be corrected before initiating therapy with Irbesartan/Hydrochlorothiazide.
Warnings
Warning 1
There is an increased risk of severe hypotension and renal insufficiency when patients with bilateral renal artery stenosis or stenosis of the artery to a single functioning kidney are treated with angiotensin converting enzyme inhibitors or angiotensin-II receptor antagonists. While this is not documented with Irbesartan/Hydrochlorothiazide , a similar effect should be anticipated.
Warning 2
Patients with primary aldosteronism generally will not respond to antihypertensive medicinal products acting through inhibition of the renin-angiotensin system. Therefore, the use of Irbesartan/Hydrochlorothiazide is not recommended.
Warning 3
Thiazides should be used with caution in patients with impaired hepatic function or progressive liver disease, since minor alterations of fluid and electrolyte balance may precipitate hepatic coma. Irbesartan/HCT must not be used in patients with severe hepatic impairment. No dosage adjustment is required in patients with mild to moderate hepatic impairment.
Additional Information
Pregnancy category
Always consult your physician before using any medicine.
Storage (YES/NO)
Store this medicine at room temperature, away from direct light and heat.
Get the latest updates on new products and upcoming sales
Thanks for subscribing!
This email has been registered!

| Product | SKU | Rating | Description | Collection | Availability | Product Type | Other Details |
|---|